|
Ferinject (ferric carboxymaltose)
1. NAME OF THE MEDICINAL PRODUCT
2. QUALITATIVE AND QUANTITATIVE COMPOSITION
3. PHARMACEUTICAL FORM
4. CLINICAL PARTICULARS
4.1 Therapeutic indications
4.2 Posology and method of administration
4.3 Contraindications
4.4 Special warnings and precautions for use
4.5 Interaction with other medicinal products and other forms of interaction
4.6 Pregnancy and lactation
4.7 Effects on ability to drive and use machines
4.8 Undesirable effects
4.9 Overdose
5. PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
5.2 Pharmacokinetic properties
5.3 Preclinical safety data
6. PHARMACEUTICAL PARTICULARS
6.1 List of excipients
6.2 Incompatibilities
6.3 Shelf life
6.4 Special precautions for storage
6.5 Nature and contents of container
6.6 Special precautions for disposal and other handling
7. MARKETING AUTHORISATION HOLDER
8. MARKETING AUTHORISATION NUMBER(S)
9. DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
10. DATE OF REVISION OF THE TEXT
|
|
Ferinject 50 mg iron/ml solution for injection/infusion. 50 mg iron/ml solution for injection/infusion.
|
|
Go to top of the page
|
|
One ml of solution contains 50 mg of iron as ferric carboxymaltose.
Each 2 ml vial contains 100 mg of iron as ferric carboxymaltose.
Each 10 ml vial contains 500 mg of iron as ferric carboxymaltose.
One ml of solution contains up to 5.5 mg (0.24 mmol) sodium, see section 4.4. For a full list of excipients, see section 6.1.
|
|
Go to top of the page
|
|
Solution for injection/infusion. Dark brown, non-transparent, aqueous solution.
|
|
Go to top of the page
Go to top of the page
|
|
Ferinject is indicated for treatment of iron deficiency when oral iron preparations are ineffective or cannot be used.
The diagnosis must be based on laboratory tests.
|
|
Go to top of the page
|
|
Determination of the cumulative iron dose
The cumulative dose for repletion of iron using Ferinject is determined based on the patient's body weight and haemoglobin level and must not be exceeded. The following table should be used to determine the cumulative iron dose:
|
Hb (g/dL)
|
Patients with body weight
35 kg to <70 kg
|
Patients with body weight
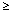 70 kg 70 kg
|
|
<10
|
1500 mg
|
2000 mg
|
|
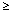 10 10
|
1000 mg
|
1500 mg
|
Note: A cumulative iron dose of 500 mg should not be exceeded for patients with body weight < 35 kg.
For overweight patients, a normal body weight/blood volume relationship should be assumed when determining the iron requirement.
For patients with an Hb value 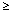 14 g/dL, an initial dose of 500 mg iron should be given and iron parameters should be checked prior to repeat dosing. 14 g/dL, an initial dose of 500 mg iron should be given and iron parameters should be checked prior to repeat dosing.
Post repletion, regular assessments should be completed to ensure that iron levels are corrected and maintained.
Maximum tolerated single dose
A single dose of Ferinject should not exceed 1000 mg of iron (20 ml) per day. Do not administer 1000 mg of iron (20 ml) more than once a week.
Intravenous injection:
Ferinject may be administered by intravenous injection using undiluted solution up to 1000 mg iron (up to a maximum of 15 mg/kg body weight). For doses greater than 200 and up to 500 mg iron, Ferinject should be administered at a rate of 100 mg/min. For doses greater than 500 and up to 1000 mg iron, Ferinject should be administered over 15 minutes.
Intravenous drip infusion:
Ferinject may be administered by intravenous infusion up to a maximum single dose of 1000 mg of iron (up to a maximum of 20 mg/kg body weight).
Method of administration
Ferinject must be administered only by the intravenous route: by bolus injection, or during a haemodialysis session undiluted directly into the venous limb of the dialyser, or by drip infusion. In case of drip infusion Ferinject must be diluted only in sterile 0.9% m/V sodium chloride solution as follows:
Dilution plan of Ferinject for intravenous drip infusion
|
Ferinject
|
Iron
|
Maximum amount of sterile 0.9% m/V sodium chloride solution
|
Minimum administration time
|
|
2
|
to
|
4 ml
|
100
|
to
|
200 mg
|
50 ml
|
-
|
|
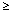 4 4
|
to
|
10 ml
|
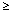 200 200
|
to
|
500 mg
|
100 ml
|
6 minutes
|
|
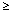 10 10
|
to
|
20 ml
|
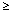 500 500
|
to
|
1000 mg
|
250 ml
|
15 minutes
|
Note: For stability reasons, dilutions to concentrations less than 2 mg iron/ml are not permissible.
Ferinject must not be administered by the subcutaneous or intramuscular route.
Haemodialysis-dependent chronic kidney disease
A single maximum daily injection dose of 200 mg iron should not be exceeded in haemodialysis-dependent chronic kidney disease patients (see also section 4.4).
Paediatric population
The use of Ferinject has not been studied in children, and therefore is not recommended in children under 14 years.
|
|
Go to top of the page
|
|
The use of Ferinject is contraindicated in cases of:
• known hypersensitivity to Ferinject or to any of its excipients
• anaemia not attributed to iron deficiency, e.g. other microcytic anaemia
• evidence of iron overload or disturbances in utilisation of iron
|
|
Go to top of the page
|
|
Parenterally administered iron preparations can cause hypersensitivity reactions including anaphylactoid reactions, which may be potentially fatal (see section 4.8). Therefore, facilities for cardio-pulmonary resuscitation must be available. If allergic reactions or signs of intolerance occur during administration, the treatment must be stopped immediately.
In patients with liver dysfunction, parenteral iron should only be administered after careful risk/benefit assessment. Parenteral iron administration should be avoided in patients with hepatic dysfunction where iron overload is a precipitating factor, in particular Porphyria Cutanea Tarda (PCT). Careful monitoring of iron status is recommended to avoid iron overload.
No safety data on haemodialysis-dependent chronic kidney disease patients receiving single doses of more than 200 mg iron are available.
Parenteral iron must be used with caution in case of acute or chronic infection, asthma, eczema or atopic allergies. It is recommended that the administration of Ferinject is stopped in patients with ongoing bacteraemia. In patients with chronic infection a risk/benefit eva luation has to be performed, taking into account the suppression of erythropoiesis.
Caution should be exercised to avoid paravenous leakage when administering Ferinject. Paravenous leakage of Ferinject at the injection site may lead to brown discolouration and irritation of the skin. In case of paravenous leakage, the administration of Ferinject must be stopped immediately.
One ml of undiluted Ferinject contains up to 5.5 mg (0.24 mmol) of sodium. This has to be taken into account in patients on a sodium-controlled diet.
One ml of undiluted Ferinject contains maximally 75 µg aluminium. This should be considered in the treatment of patients undergoing dialysis.
The use of Ferinject has not been studied in children.
Do not administer 20 ml (1000 mg of iron) as an injection or infusion more than once a week.
|
|
Go to top of the page
|
|
As with all parenteral iron preparations the absorption of oral iron is reduced when administered concomitantly. Therefore, if required, oral iron therapy should not be started for at least 5 days after the last injection of Ferinject.
|
|
Go to top of the page
|
|
Pregnancy
There are no data from the use of Ferinject in pregnant women. A careful risk/benefit eva luation is required before use during pregnancy and Ferinject should not be used during pregnancy unless clearly necessary. Animal data suggest that iron released from Ferinject can cross the placental barrier and that its use during pregnancy may influence skeletal development in the fetus (see section 5.3).
Iron deficiency occurring in the first trimester of pregnancy can in many cases be treated with oral iron. If the benefit of Ferinject treatment is judged to outweigh the potential risk to the fetus, it is recommended that treatment should be confined to the second and third trimester.
Lactation
Clinical studies showed that transfer of iron from Ferinject to human milk was negligible (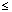 1%). Based on limited data on nursing women it is unlikely that Ferinject represents a risk to the nursing child. 1%). Based on limited data on nursing women it is unlikely that Ferinject represents a risk to the nursing child.
Fertility
There are no data on the effect of Ferinject on human fertility. Fertility was unaffected following Ferinject treatment in animal studies (see section 5.3).
|
|
Go to top of the page
|
|
Ferinject is unlikely to impair the ability to drive or operate machines.
|
|
Go to top of the page
|
|
The most commonly reported ADR is headache, occurring in 3.3% of the patients.
|
System Organ Class
|
Very common (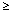 1/10) 1/10)
|
Common (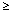 1/100, <1/10) 1/100, <1/10)
|
Uncommon (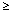 1/1000, <1/100) 1/1000, <1/100)
|
Rare (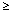 1/10000, <1/1000) 1/10000, <1/1000)
|
|
Immune system disorders
|
|
|
Hypersensitivity including anaphylactoid reactions
|
|
|
Nervous system disorders
|
|
Headache, dizziness
|
Paraesthesia
|
|
|
Vascular disorders
|
|
|
Hypotension, hypertension, flushing
|
|
|
Respiratory, thoracic and mediastinal disorders
|
|
|
|
Dyspnoea
|
|
Gastrointestinal disorders
|
|
Nausea, abdominal pain, constipation, diarrhoea
|
Dysgeusia, vomiting, dyspepsia, flatulence
|
|
|
Skin and subcutaneous tissue disorders
|
|
Rash
|
Pruritus, urticaria
|
|
|
Musculoskeletal and connective tissue disorders
|
|
|
Myalgia, back pain, arthralgia
|
|
|
General disorders and administration site conditions
|
|
Injection Site Reactions
|
Pyrexia, fatigue, chest pain, rigors, malaise, oedema peripheral
|
|
|
Investigations
|
|
Transient blood phosphorus decreased, alanine aminotransferase increased
|
Aspartate aminotransferase increased, gamma-glutamyltransferase increased, blood lactate dehydrogenase increased
|
|
There are no undesirable effects with unknown frequency.
|
|
Go to top of the page
|
|
Administration of Ferinject in quantities exceeding the amount needed to correct iron deficit at the time of administration may lead to accumulation of iron in storage sites eventually leading to haemosiderosis. Monitoring of iron parameters such as serum ferritin and transferrin saturation may assist in recognising iron accumulation. If iron accumulation has occurred, the use of an iron chelator may be considered.
|
|
Go to top of the page
Go to top of the page
|
|
Pharmacotherapeutic group: Iron trivalent, parenteral preparation
ATC Code: B03A C01
Ferinject solution for injection/infusion contains iron in a stable ferric state as a complex of a polynuclear iron-hydroxide core with a carbohydrate ligand. The complex is designed to provide, in a controlled way, utilisable iron for the iron transport and storage proteins in the body (transferrin and ferritin, respectively). Clinical studies showed that the haematological response and the filling of the iron stores was faster after intravenous administration of Ferinject than with orally administered comparators.
Using positron emission tomography (PET) it was demonstrated that red cell utilisation of 59Fe and 52Fe from radio-labelled Ferinject ranged from 61% to 99%. After 24 days, patients with iron deficiency showed utilisation of radio-labelled iron of 91% to 99% and patients with renal anaemia showed utilisation of radio-labelled iron of 61% to 84%.
|
|
Go to top of the page
|
|
Using positron emission tomography (PET) it was demonstrated that 59Fe and 52Fe from Ferinject was rapidly eliminated from the blood, transferred to the bone marrow, and deposited in the liver and spleen.
After administration of a single dose of Ferinject of 100 to 1000 mg of iron in iron deficient patients, maximum total serum iron levels of 37 µg/ml up to 333 µg/ml after 15 minutes to 1.21 hours respectively are obtained. The volume of the central compartment corresponds well to the volume of the plasma (approximately 3 litres).
The iron injected or infused was rapidly cleared from the plasma, the terminal half-life ranged from 7 to 12 hours, the mean residence time (MRT) from 11 to 18 hours. Renal elimination of iron was negligible.
|
|
Go to top of the page
|
|
Pre-clinical data revealed no special hazard for humans based on conventional studies of safety pharmacology, repeat dose toxicity and genotoxicity. Pre-clinical studies indicate that iron released from Ferinject does cross the placental barrier and is excreted in milk in limited, controlled amounts. In reproductive toxicology studies using iron replete rabbits Ferinject was associated with minor skeletal abnormalities in the fetus. In a fertility study in rats, there were no effects on fertility for either male or female animals. No long-term studies in animals have been performed to eva luate the carcinogenic potential of Ferinject. No evidence of allergic or immunotoxic potential has been observed. A controlled in-vivo test demonstrated no cross-reactivity of Ferinject with anti-dextran antibodies. No local irritation or intolerance was observed after intravenous administration.
|
|
Go to top of the page
Go to top of the page
|
|
Sodium hydroxide (for pH adjustment)
Hydrochloric acid (for pH adjustment)
Water for injections
|
|
Go to top of the page
|
|
This medicinal product must not be mixed with other medicinal products except those mentioned in section 6.6.
The compatibility with containers other than polyethylene and glass is not known.
|
|
Go to top of the page
|
|
Shelf-life of the product as packaged for sale:
3 years.
Shelf-life after first opening of the container:
From a microbiological point of view, preparations for parenteral administration should be used immediately.
Shelf-life after dilution with sterile 0.9% m/V sodium chloride solution:
From a microbiological point of view, preparations for parenteral administration should be used immediately after dilution with sterile 0.9% m/V sodium chloride solution.
|
|
Go to top of the page
|
|
Store in the original package. Do not store above 30 °C. Do not freeze.
|
|
Go to top of the page
|
|
2 ml of solution in a vial (type I glass) with bromobutyl rubber stopper and aluminium cap in pack sizes of 1 and 5 vials.
10 ml of solution in a vial (type I glass) with bromobutyl rubber stopper and aluminium cap in pack sizes of 1 and 5 vials.
Not all pack sizes may be marketed.
|
|
Go to top of the page
|
|
Inspect vials visually for sediment and damage before use. Use only those containing sediment-free, homogeneous solution.
Each vial of Ferinject is intended for single use only. Any unused product or waste material should be disposed of in accordance with local requirements.
Ferinject must only be mixed with sterile 0.9% m/V sodium chloride solution. No other intravenous dilution solutions and therapeutic agents should be used, as there is the potential for precipitation and/or interaction. For dilution instructions, see section 4.2.
 
|
|
Go to top of the page
|
|
Vifor France SA
7-13, Boulevard Paul-Emile Victor
92200 Neuilly-sur-Seine
|
|
|



