HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use AURYXIA safely and effectively. See full prescribing information for AURYXIA. AURYXIA (ferric citrate) tablets, for ...
These highlights do not include all the information needed to use AURYXIA safely and effectively. See full prescribing information for AURYXIA.
AURYXIA (ferric citrate) tablets, for oral use
Initial U.S. Approval: 2014
INDICATIONS AND USAGE
Auryxia™ is a phosphate binder indicated for the control of serum phosphorus levels in patients with chronic kidney disease on dialysis (
1)
DOSAGE AND ADMINISTRATION
-
Starting dose is 2 tablets orally 3 times per day with meals (2)
-
Adjust dose by 1 to 2 tablets as needed to maintain serum phosphorus at target levels, up to a maximum of 12 tablets daily. Dose can be titrated at 1-week or longer intervals. (2)
DOSAGE FORMS AND STRENGTHS
-
Tablets: 210 mg ferric iron, equivalent to 1 g ferric citrate (3)
CONTRAINDICATIONS
-
Iron overload syndromes (e.g., hemochromatosis) (4)
WARNINGS AND PRECAUTIONS
-
Iron overload: Monitor ferritin and TSAT. Patients may require a reduction in dose or discontinuation of IV iron. (5.1)
-
Accidental overdose of iron-containing products is a leading cause of fatal poisoning in children under 6 years of age. Keep this product out of reach of children. In case of accidental overdose, call a doctor or poison control center immediately. (5.2)
-
Patients with gastrointestinal bleeding or inflammation: Safety has not been established. (5.3)
ADVERSE REACTIONS
-
In clinical trials, likely adverse reactions occurring with Auryxia included diarrhea, discolored feces, constipation, nausea, and vomiting (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Keryx Biopharmaceuticals at 1-844-445-3799 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
DRUG INTERACTIONS
-
When clinically significant drug interactions are expected, consider separation of the timing of administration. Consider monitoring clinical responses or blood levels of the concomitant medication (7)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 11/2016
FULL PRESCRIBING INFORMATION: CONTENTS*
Table of Contents
1 INDICATIONS AND USAGE
Auryxia (ferric citrate) is a phosphate binder indicated for the control of serum phosphorus levels in patients with chronic kidney disease on dialysis.
Auryxia (ferric citrate) is a phosphate binder indicated for the control of serum phosphorus levels in patients with chronic kidney disease on dialysis.
2 DOSAGE AND ADMINISTRATION
2.1 Dosing and Dose Adjustment - The recommended starting dose is 2 tablets orally 3 times per day with meals. Serum phosphorus levels should be monitored and the ...
2.1 Dosing and Dose Adjustment
The recommended starting dose is 2 tablets orally 3 times per day with meals. Serum phosphorus levels should be monitored and the dose of Auryxia titrated in decrements or increments of 1 to 2 tablets per day as needed to maintain serum phosphorus at target levels, up to a maximum dose of 12 tablets daily. Dose can be titrated at 1-week or longer intervals.
In a clinical trial conducted in the United States, patients required an average of 8 to 9 tablets a day to control serum phosphorus levels.
3 DOSAGE FORMS AND STRENGTHS
Tablet: Auryxia 210 mg ferric iron, equivalent to 1 g ferric citrate, film-coated, peach-colored, and oval-shaped tablet debossed with “KX52”.
Tablet: Auryxia 210 mg ferric iron, equivalent to 1 g ferric citrate, film-coated, peach-colored, and oval-shaped tablet debossed with “KX52”.
4 CONTRAINDICATIONS
Auryxia is contraindicated in patients with iron overload syndromes (e.g., hemochromatosis) [see Warnings and Precautions (5.1)].
Auryxia is contraindicated in patients with iron overload syndromes (e.g., hemochromatosis) [see Warnings and Precautions (5.1)].
5 WARNINGS AND PRECAUTIONS
5.1 Iron Overload - Iron absorption from Auryxia may lead to excessive elevations in iron stores. Increases in serum ferritin and transferrin saturation (TSAT) levels ...
5.1 Iron Overload
Iron absorption from Auryxia may lead to excessive elevations in iron stores. Increases in serum ferritin and transferrin saturation (TSAT) levels were observed in clinical trials. In a 56-week safety and efficacy trial in which concomitant use of Auryxia and IV iron was permitted, 55 (19%) of patients treated with Auryxia had a ferritin level >1500 ng/mL as compared with 13 (9%) of patients treated with active control.
Assess iron parameters (e.g., serum ferritin and TSAT) prior to initiating Auryxia and monitor iron parameters while on therapy [see Contraindications (4), Overdosage (10) and Clinical Pharmacology (12.2)]. Patients receiving IV iron may require a reduction in dose or discontinuation of IV iron therapy.
5.2 Accidental Overdose of Iron
Accidental overdose of iron-containing products is a leading cause of fatal poisoning in children under 6 years of age. Keep this product out of the reach of children. In case of accidental overdose, call a doctor or poison control center immediately.
5.3 Patients with Gastrointestinal Bleeding or Inflammation
Patients with inflammatory bowel disease or active, symptomatic gastrointestinal bleeding were excluded from clinical trials. Safety has not been established in these populations.
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a ...
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to adverse reaction rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Adverse reactions to a drug are most readily ascertained by comparison with placebo, but there is little placebo-controlled experience with Auryxia, so this section describes adverse events with Auryxia, some of which may be disease-related, rather than treatment-related.
A total of 289 patients were treated with Auryxia and 149 patients were treated with active control (sevelamer carbonate and/or calcium acetate) during the 52-week, randomized, open-label, active control phase of a trial in patients on dialysis. A total of 322 patients were treated with Auryxia for up to 28 days in three short-term trials. Across these trials, 557 unique patients were treated with Auryxia; dosage regimens in these trials ranged from 210 mg to 2,520 mg of ferric iron per day, equivalent to 1 to 12 tablets of Auryxia. In these trials, adverse events reported for Auryxia were similar to those reported for the active control group.
Adverse events reported in more than 5% of patients treated with Auryxia in these trials included diarrhea (21%), nausea (11%), constipation (8%), vomiting (7%), and cough (6%).
During the 52-week, active-control period, 60 patients (21%) on Auryxia discontinued study drug because of an adverse event, as compared to 21 patients (14%) in the active control arm. Patients who were previously intolerant to any of the active control treatments (calcium acetate and sevelamer carbonate) were not eligible to enroll in the study. Gastrointestinal adverse reactions were the most common reason for discontinuing Auryxia (14%).
Auryxia is associated with discolored feces (dark stools) related to the iron content, but this staining is not clinically relevant and does not affect laboratory tests for occult bleeding, which detect heme rather than non-heme iron in the stool.
7 DRUG INTERACTIONS
Table 1: Oral drugs that can be administered concomitantly with Auryxia - Amlodipine - Aspirin - Atorvastatin ...
Oral medications not listed in Table 1
There are no empirical data on avoiding drug interactions between Auryxia and most concomitant oral drugs. For oral medications where a reduction in the bioavailability of that medication would have a clinically significant effect on its safety or efficacy, consider separation of the timing of the administration of the two drugs. The duration of separation depends upon the absorption characteristics of the medication concomitantly administered, such as the time to reach peak systemic levels and whether the drug is an immediate release or an extended release product. Consider monitoring clinical responses or blood levels of concomitant medications that have a narrow therapeutic range.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy - Pregnancy Category B: There are no adequate and well-controlled studies in pregnant women. It is not known whether Auryxia can cause fetal harm when ...
8.1 Pregnancy
Pregnancy Category B: There are no adequate and well-controlled studies in pregnant women. It is not known whether Auryxia can cause fetal harm when administered to a pregnant woman. Animal reproduction studies have not been conducted.
The effect of Auryxia on the absorption of vitamins and other nutrients has not been studied in pregnant women. Requirements for vitamins and other nutrients are increased in pregnancy. An overdose of iron in pregnant women may carry a risk for spontaneous abortion, gestational diabetes and fetal malformation [see Nonclinical Toxicology (13.1)].
8.2 Labor and Delivery
The effects of Auryxia on labor and delivery are unknown.
8.3 Nursing Mothers
Data from rat studies have shown the transfer of iron into milk by divalent metal transporter-1 (DMT-1) and ferroportin-1 (FPN-1). Hence, there is a possibility of infant exposure when Auryxia is administered to a nursing woman.
8.4 Pediatric Use
The safety and efficacy of Auryxia have not been established in pediatric patients.
8.5 Geriatric Use
Clinical studies of Auryxia included 106 subjects aged 65 years and older (33 subjects aged 75 years and older). Overall, the clinical study experience has not identified any obvious differences in responses between the elderly and younger patients in the tolerability or efficacy of Auryxia.
10 OVERDOSAGE
No data are available regarding overdose of Auryxia in patients. In patients with chronic kidney disease on dialysis, the maximum dose studied was 2,520 mg ferric iron (12 tablets of ...
No data are available regarding overdose of Auryxia in patients. In patients with chronic kidney disease on dialysis, the maximum dose studied was 2,520 mg ferric iron (12 tablets of Auryxia) per day. Iron absorption from Auryxia may lead to excessive elevations in iron stores, especially when concomitant IV iron is used [see Warnings and Precautions (5.1)].
In clinical trials, one case of elevated iron in the liver as confirmed by biopsy was reported in a patient administered IV iron and Auryxia.
Because accidental overdose of iron-containing products is a leading cause of fatal poisoning in children under 6 years of age, this product must be kept out of the reach of children. In case of accidental overdose, a doctor or poison control center should be contacted immediately [see Warnings and Precautions (5.2)].
11 DESCRIPTION
Auryxia (ferric citrate) is known chemically as iron (+3), x (1, 2, 3-propanetricarboxylic acid, 2 hydroxy-), y (H2O) Auryxia 210 mg ferric iron tablets, equivalent to 1g ferric ...
Auryxia (ferric citrate) is known chemically as iron (+3), x (1, 2, 3-propanetricarboxylic acid, 2 hydroxy-), y (H2O)
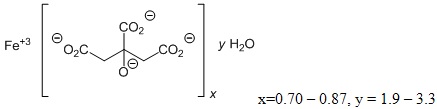
Auryxia 210 mg ferric iron tablets, equivalent to 1g ferric citrate, are film-coated, peach-colored, and oval-shaped tablets debossed with “KX52”. The inactive ingredients are pregelatinized starch and calcium stearate. In addition, the film-coating contains the following inactive ingredients; hypromellose, titanium dioxide, triacetin, and FD&C Yellow #6/Sunset Yellow FCF Aluminum Lake, FD&C Red #40/Allura Red AC Aluminum Lake, and FD&C Blue #2/Indigo Carmine Aluminum Lake.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action - Ferric iron binds dietary phosphate in the GI tract and precipitates as ferric phosphate. This compound is insoluble and is excreted in the ...
12.1 Mechanism of Action
Ferric iron binds dietary phosphate in the GI tract and precipitates as ferric phosphate. This compound is insoluble and is excreted in the stool. By binding phosphate in the GI tract and decreasing absorption, ferric citrate lowers the phosphate concentration in the serum.
12.2 Pharmacodynamics
In addition to effects on serum phosphorus levels, Auryxia has been shown to increase serum iron parameters, including ferritin, iron and TSAT. In dialysis patients treated with Auryxia in a 52-week study in which IV iron could also be administered, mean (SD) ferritin levels rose from 593 (293) ng/mL to 895 (482) ng/mL, mean (SD) TSAT levels rose from 31% (11) to 39% (17) and mean (SD) iron levels rose from 73 (29) mcg/dL to 88 (42) mcg/dL. In contrast, in patients treated with active control, these parameters remained relatively constant [see Contraindications (4) and Warnings and Precautions (5.1)].
12.3 Pharmacokinetics
Absorption and Distribution
Formal pharmacokinetic studies have not been performed with Auryxia. Examination of serum iron parameters has shown that there is systemic absorption of iron from Auryxia [see Contraindications (4), Warnings and Precautions (5.1) and Clinical Pharmacology (12.2)].
Drug Interaction Studies
In vitro
Of the drugs screened for an interaction with ferric citrate in vitro, only doxycycline showed the potential for interaction with at least 70% decrease in its concentration. This interaction can be avoided by spacing the administration of doxycycline and ferric citrate [see Drug Interactions (7)].
In vivo
Six drug interaction studies (N=26-60/study) were conducted to establish the effects of Auryxia (administered as 3 x 2 g/day with meals) on the disposition of concomitantly orally administered clopidogrel, ciprofloxacin, digoxin, diltiazem, glimepiride and losartan in healthy subjects. With the exception of ciprofloxacin, Auryxia did not alter the systemic exposure of the tested drugs, as measured by the area under the curve (AUC) and Cmax of the tested drugs when either co-administered with Auryxia or given 2 hours later. Auryxia decreased the relative bioavailability of concomitantly administered ciprofloxacin by approximately 45%. However, there was no interaction when Auryxia and ciprofloxacin were taken 2 hours apart. Consequently, ciprofloxacin should be taken at least 2 hours before or after Auryxia is dosed [see Drug Interactions (7)].
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Data from carcinogenesis studies have shown that ferric citrate is not carcinogenic in mice and rats when ...
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Data from carcinogenesis studies have shown that ferric citrate is not carcinogenic in mice and rats when administered intramuscularly or subcutaneously. Ferric citrate was neither mutagenic in the bacterial reverse mutation assay (Ames test) nor clastogenic in the chromosomal aberration test in Chinese hamster fibroblasts.
The potential for ferric citrate to impair reproductive performance or to cause fetal malformation has not been eva luated. Skeletal and encephalic malformation was observed in neonatal mice when ferric gluconate was administered intraperitoneally to gravid dams on gestation days 7-9. However, oral administration of other ferric or ferrous compounds to gravid CD1-mice and Wistar-rats caused no fetal malformation.
14 CLINICAL STUDIES
The ability of Auryxia to lower serum phosphorus in patients with CKD on dialysis was demonstrated in randomized clinical trials: one 56-week, safety and efficacy trial, consisting of a ...
The ability of Auryxia to lower serum phosphorus in patients with CKD on dialysis was demonstrated in randomized clinical trials: one 56-week, safety and efficacy trial, consisting of a 52-week active-controlled phase and a 4-week, placebo-controlled, randomized withdrawal period, and one 4-week open-label trial of different fixed doses of Auryxia. Both trials excluded subjects who had an absolute requirement for aluminum containing drugs with meals.
14.1 Long-term, Randomized, Controlled, Safety and Efficacy Trial
After the 2-week washout period during which phosphate binders were held, patients with a mean serum phosphorus of 7.5 mg/dL during washout were randomized 2:1 to Auryxia (N=292) or active control (calcium acetate and/or sevelamer carbonate; N=149). The majority (>96%) of subjects were on hemodialysis. The starting dose of Auryxia was 6 tablets/day, divided with meals. The starting dose of active control was the patient’s dose prior to the washout period. The dose of phosphate binder was increased or decreased as needed to maintain serum phosphorus levels between 3.5 and 5.5 mg/dL, to a maximum of 12 tablets/day.
As shown in the figure below, serum phosphorus levels declined following initiation of therapy. The phosphorus lowering effect was maintained over 52 weeks of treatment.
Figure 1: Serum Phosphorus Control over 52 Weeks
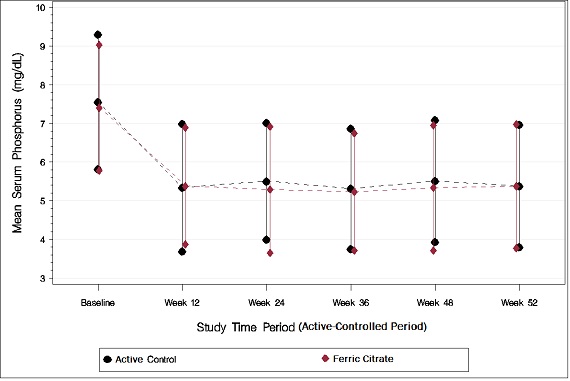
Following completion of the 52-week active-controlled phase, Auryxia-treated patients were eligible to enter a 4-week placebo-controlled randomized withdrawal phase, in which patients were re-randomized in a 1:1 ratio to receive Auryxia (N=96) or placebo (N=96). During the placebo-controlled period, the serum phosphorus concentration rose by 2.2 mg/dL on placebo relative to patients who remained on Auryxia.
14.2 Fixed-Dose Trial
Following a 1- to 2-week washout from all phosphate-binding agents, 154 patients with hyperphosphatemia (mean serum phosphorus of 7.5 mg/dL) and CKD on dialysis were randomized in a 1:1:1 ratio to 1, 6, or 8 tablets/day of Auryxia for 4 weeks. Auryxia was administered with meals; subjects receiving 1 tablet/day were instructed to take it with their largest meal of the day, and subjects on 6 or 8 tablets/day took divided doses in any distribution with meals. Dose-dependent decreases in serum phosphorus were observed by Day 7 and remained relatively stable for the duration of treatment. The demonstrated reductions from baseline to Week 4 in mean serum phosphorus were significantly greater with 6 and 8 tablets/day than with 1 tablet/day (p<0.0001). Mean reduction in serum phosphorus at Week 4 was 0.1 mg/dL with 1 tablet/day, 1.9 mg/dL with 6 tablets/day, and 2.1 mg/dL with 8 tablets/day.
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied - Tablets: Auryxia 210 mg ferric iron tablets equivalent to 1 g of ferric citrate are supplied as 200 tablets in 400-cc high-density polyethylene ...
16.1 How Supplied
Tablets: Auryxia 210 mg ferric iron tablets equivalent to 1 g of ferric citrate are supplied as 200 tablets in 400-cc high-density polyethylene bottles. The 210 mg ferric iron tablets are film-coated, peach-colored, and oval-shaped tablets debossed with “KX52.”
1 Bottle of 200-count 210 mg ferric iron tablets (NDC 59922-631-01)
16.2 Storage and Handling
Storage: Store at 20 to 25°C (68 to 77°F): excursions permitted to 15° to 30°C (59°F to 86°F) [See USP controlled room temperature]. Protect from moisture.
17 PATIENT COUNSELING INFORMATION
17.1 Dosing Recommendations - Inform patients to take Auryxia as directed with meals and adhere to their prescribed diets. Instruct patients on concomitant ...
17.1 Dosing Recommendations
Inform patients to take Auryxia as directed with meals and adhere to their prescribed diets. Instruct patients on concomitant medications that should be dosed apart from Auryxia.
17.2 Adverse Reactions
Advise patients that Auryxia may cause discolored (dark) stools, but this staining of the stool is considered normal with oral medications containing iron.
Auryxia may cause diarrhea, nausea, constipation and vomiting. Advise patients to report severe or persistent gastrointestinal symptoms to their physician.
Manufactured for and Distributed by:
Keryx Biopharmaceuticals, Inc.
One Marina Park Drive, 12th Floor Boston, MA 02210, US
Issued 11/2016 Rev 3.3
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - NDC: 59922-631-01 - 210 mg 200-ct Bottle
PRINCIPAL DISPLAY PANEL - NDC: 59922-631-01 - 210 mg 200-ct Bottle

PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - NDC: 59922-631-91 - 210 mg 200-ct Professional Samples Bottle
PRINCIPAL DISPLAY PANEL - NDC: 59922-631-91 - 210 mg 200-ct Professional Samples Bottle

PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - NDC: 59922-631-92 - 210 mg 50-ct Professional Samples Bottle
PRINCIPAL DISPLAY PANEL - NDC: 59922-631-92 - 210 mg 50-ct Professional Samples Bottle

INGREDIENTS AND APPEARANCE
Product Information



