LOVAZA - omega-3-acid ethyl esters capsule, liquid filled
SmithKline Beecham Corporation
LOVAZA® (omega-3-acid ethyl esters) Capsules
DESCRIPTION
LOVAZA, a lipid-regulating agent, is supplied as a liquid-filled gel capsule for oral administration. Each 1-gram capsule of LOVAZA (omega-3-acid ethyl esters) contains at least 900 mg of the ethyl esters of omega-3 fatty acids. These are predominantly a combination of ethyl esters of eicosapentaenoic acid (EPA - approximately 465 mg) and docosahexaenoic acid (DHA - approximately 375 mg).
The structural formula of EPA ethyl ester is:
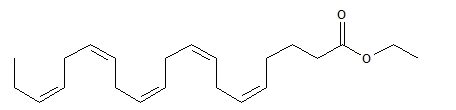
The empirical formula of EPA ethyl ester is C22H34O2, and the molecular weight of EPA ethyl ester is 330.51.
The structural formula of DHA ethyl ester is:

The empirical formula of DHA ethyl ester is C24H36O2, and the molecular weight of DHA ethyl ester is 356.55.
LOVAZA capsules also contain the following inactive ingredients: 4 mg α-tocopherol (in a carrier of partially hydrogenated vegetable oils including soybean oil), and gelatin, glycerol, and purified water (components of the capsule shell).
CLINICAL PHARMACOLOGY
Mechanism of Action
The mechanism of action of LOVAZA is not completely understood. Potential mechanisms of action include inhibition of acyl CoA:1,2-diacylglycerol acyltransferase, increased mitochondrial and peroxisomal β-oxidation in the liver, decreased lipogenesis in the liver, and increased plasma lipoprotein lipase activity. LOVAZA may reduce the synthesis of triglycerides (TGs) in the liver because EPA and DHA are poor substrates for the enzymes responsible for TG synthesis, and EPA and DHA inhibit esterification of other fatty acids.
Pharmacokinetic and Bioavailability Studies
In healthy volunteers and in patients with hypertriglyceridemia (HTG), EPA and DHA were absorbed when administered as ethyl esters orally. Omega-3-acids administered as ethyl esters (LOVAZA) induced significant, dose–dependent increases in serum phospholipid EPA content, though increases in DHA content were less marked and not dose-dependent when administered as ethyl esters. Uptake of EPA and DHA into serum phospholipids in subjects t reated with LOVAZA was independent of age (<49 years versus ≥49 years). Females tended to have more uptake of EPA into serum phospholipids than males. Pharmacokinetic data on LOVAZA in children are not available.
Drug Interactions
Cytochrome P450-Dependent Monooxygenase Activities
The effect of a mixture of free fatty acids (FFA), EPA/DHA and their FFA-albumin conjugate on cytochrome P450-dependent monooxygenase activities was assessed in human liver microsomes. At the 23 micromole concentration, FFA resulted in a less than 32% inhibition of CYP1A2, 2A6, 2C9, 2C19, 2D6, 2E1, and 3A. At the 23 micromole concentration, the FFA-albumin conjugate resulted in a less than 20% inhibition of CYP2A6, 2C19, 2D6, and 3A, with a 68% inhibition being seen for CYP2E1. Since the free forms of the EPA and DHA are undetectable in the circulation (<1 micromole), clinically significant drug-drug interactions due to inhibition of P450-mediated metabolism EPA/DHA combinations are not expected in humans.
CLINICAL STUDIES
High Triglycerides
Add-on to HMG-CoA reductase inhibitor therapy
The effects of LOVAZA 4 g per day as add-on therapy to treatment with simvastatin were eva luated in a randomized, placebo-controlled, double-blind, parall



