2014年7月23日,欧洲动脉粥样硬化学会(EAS)发布了最新的纯合子家族性高胆固醇血症治疗指南,旨在帮助临床医生检测和管理纯合子家族性高胆固醇血症(HoFH)患者。全文发表于《Eur Heart J 2014》。
流行病学
HoFH是一种非常罕见的遗传性疾病,其特点是:LDL-C水平升高和早发动脉粥样硬化性心血管疾病风险升高。杂合子家族性高胆固醇血症(HeFH)的发病率为1/500,而HoFH非常罕见。尽管没有HoFH的流行病学数据,但根据历史资料估计其发病率为1/100万;近期的一般人群研究显示,该病的患病率为1/16-30万。EAS指出,若不加以干预,HoFH患者通常于20岁左右发生动脉粥样硬化性心血管疾病,30岁左右死亡。
管理目标
EAS共识组指出,HoFH患者管理的目标是:早期预防动脉粥样硬化、全面控制高胆固醇血症、早期发现并发症、特别关注冠状动脉开口闭塞和主动脉瓣狭窄。不幸的是,通常在冠状动脉疾病粥样硬化比较明显时才发现HoFH,因此我们强调在儿童时期优化治疗。
诊断标准
包括主席John Chapman博士和Henry Ginsberg博士在内的共识组成员指出,基因确认可以作为HoFH的诊断标准,但未经治疗时LDL-C>500 mg/dL或经过治疗时LDL-C>300 mg/dL、皮肤或肌腱黄瘤、年龄<10岁也足以做出诊断。此外,未经治疗的LDL-C水平>500 mg/dL、双亲均为HoFH也可以诊断该病。

监测与评估
一旦诊断为HoFH,需要定期监测,因为患者早发严重动脉粥样硬化性心血管疾病的风险非常高。指南建议,给予患者全面的心血管评估,随后每年对心脏和主动脉进行多普勒超声心动图评价。如果可行的话,每5年进行一次CT冠状动脉造影检查;若有临床指征可以增加检查频次,但也要考虑辐射情况和炎临床疾病的严重程度。
治疗:现状与展望
HoFH的治疗包括生活方式改变、他汀类药物(可加依折麦布)和脂蛋白置换,上述方法联合使用,尽早开始治疗。LDL置换可以在5随时开始,最晚不超过8岁。
LDL-C治疗目标成人为<100 mg/dL,成人伴临床心血管疾病为70 mg/dL,儿童为130 mg/dL。EAS共识组认为这些目标值的设定比较严格,尽管一些新药可以帮助患者接近上述目标值。
HoFH治疗流程图:
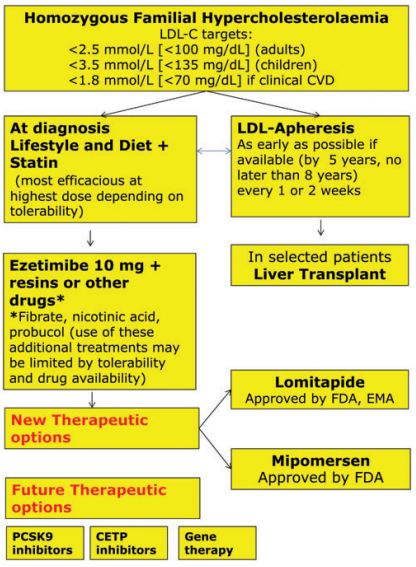
两种降LDL-C新药——lomitapide和mipomersen可以作为未达标高危患者的辅助治疗药物。尚在开发中的降LDL-C药物还有PCSK9抑制剂,例如evolocumab、alirocumab和bococizumab等。此外,CETP抑制剂,如evacetrapib和anacetrapib也被证明可以降低LDL-C水平。
编译自:Michael O'Riordan. EAS Issues New Guidance on Treating Rare Homozygous FH. Heartwire. July 24, 2014
美國 JUXTAPID® (lomitapide) capsules 歐洲 Lojuxta(lomitapide capsules)
Indication and Important Safety Information
Indication and Important Safety Information
JUXTAPID is used along with a low-fat diet and other lipid-lowering treatments, including LDL apheresis where available, in people with HoFH. It is not known whether JUXTAPID can decrease problems from high cholesterol, such as heart attack, stroke, death or other health problems. It is also not known whether Juxtapid is safe for use in people with high cholesterol but who do not have HoFH.
IMPORTANT SAFETY INFORMATION
JUXTAPID can cause serious side effects, including liver problems. For this reason, your doctor should do blood tests to check your liver before you start JUXTAPID, if your dose is increased, and while you take JUXTAPID. If your tests show some liver problems, your doctor may adjust your dose of JUXTAPID or stop it altogether.
You should tell your doctor if you have had liver problems, including liver problems while taking other medicines.
JUXTAPID is available only through certified pharmacies that are enrolled in the JUXTAPID REMS Program. Your doctor must be enrolled and certified in the program in order for you to be prescribed JUXTAPID.
You should not take JUXTAPID if:
You are pregnant
You are taking medications known as moderate or strong CYP3A4 inhibitors, which affect how the body breaks down JUXTAPID
You have moderate or severe liver problems or active liver disease, including elevated liver function tests
JUXTAPID may cause harm to your unborn baby. If you are pregnant, think you may be pregnant, or are planning to become pregnant, do not take JUXTAPID. Do not have sex while taking JUXTAPID unless you are using effective birth control. If you become pregnant while taking JUXTAPID, stop taking JUXTAPID and call your doctor right away.
Diarrhea, nausea/vomiting, and stomach pain/discomfort are very common with JUXTAPID. Strictly following a low-fat diet may help lower the chance of having these symptoms. These side effects can also be symptoms of liver problems. Tell your doctor right away if you have any of these symptoms of liver problems while taking JUXTAPID: nausea; vomiting or stomach pain that gets worse, does not go away, or changes; fever; yellowing of your eyes or skin; feeling more tired than usual; having flu-like symptoms.
JUXTAPID makes it harder for some nutrients to get into your body. Take Vitamin E and fatty acids each day while you take JUXTAPID. Ask your doctor, nurse, or dietician how to add them to your diet.
The most common side effects of JUXTAPID include diarrhea (loose stool),
nausea, vomiting, stomach cramping or pain, indigestion, and gas. Tell your doctor if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of JUXTAPID. For more information, ask your doctor or pharmacist.
Tell your doctor about all the medicines you take, including prescription and non-prescription medicines, vitamins, and herbal supplements. JUXTAPID may affect the way other medicines work, and other medicines may affect how JUXTAPID works. You should not drink grapefruit juice while taking JUXTAPID.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch, or call 1-800-FDA-1088.
http://www.juxtapid.com/
Approval Letter(s) (PDF)
Summary Review (PDF)
Officer/Employee List (PDF)
Printed Labeling (PDF)
REMS (PDF)
Medical Review(s) (PDF)
Chemistry Review(s) (PDF)
Pharmacology Review(s) (PDF)
Statistical Review(s) (PDF)
Clinical Pharmacology Biopharmaceutics Review(s) (PDF)
Risk Assessment and Risk Mitigation Review(s) (PDF)
Proprietary Name Review(s) (PDF)
Other Review(s) (PDF)
Administrative Document(s) & Correspondence (PDF)
Lojuxta : EPAR - Procedural steps taken and scientific information after authorisation
Lojuxta : EPAR - Public assessment report
CHMP summary of positive opinion for Lojuxta



